Look out for lewis structure for h2co
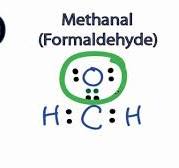
Curious lewis structure for h2co minds and chemistry enthusiasts, are you ready to dive into the fascinating world of Lewis Structures? Buckle up as we unravel the mysteries behind these essential diagrams that help us visualize molecular structures with precision. Today, our spotlight shines on H2CO, better known as formaldehyde. Get ready to sharpen your pencils and follow along as we guide you through drawing the Lewis Structure for this compound step by step. Let’s embark on this chemical adventure together!
What is Lewis Structure?
Lewis Structures are like molecular blueprints, providing a visual representation of how atoms are bonded in a compound. These diagrams help chemists predict the geometry and properties of molecules. Named after American chemist Gilbert N. Lewis, they depict the arrangement of valence electrons around atoms.
In Lewis Structures, each atom is represented by its chemical symbol surrounded by dots or lines that symbolize valence electrons. The goal is to achieve octet rule satisfaction for most elements, where atoms strive to have eight electrons in their outer shell for stability.
By understanding Lewis Structures, chemists can predict molecular shapes and reactivity patterns based on electron distribution. This tool is crucial in organic chemistry for designing new compounds and understanding reaction mechanisms.
Mastering Lewis Structures opens doors to unraveling the complex world of chemical bonding and molecular interactions with clarity and precision.
Why is it important in chemistry?
Understanding the structure of molecules is crucial in chemistry. Lewis Structures provide a visual representation of how atoms are bonded together, helping chemists predict the behavior and properties of compounds. By following specific rules, such as the octet rule, Lewis Structures allow us to determine the number of valence electrons each atom has and how they are shared in a molecule.
This knowledge is fundamental for studying chemical reactions, understanding molecular shapes, and predicting polarity. Without grasping these concepts, it would be challenging to comprehend why certain substances react with each other or why some molecules have distinct geometries.
In essence, mastering Lewis Structures opens up a whole new world within chemistry where we can explore the intricacies of atomic bonding and molecular interactions on a deeper level.
Understanding the basics of H2CO (Formaldehyde)
Formaldehyde, known by its chemical formula H2CO, is a simple yet essential compound in chemistry. It consists of one carbon atom bonded to two hydrogen atoms and an oxygen atom. This molecule plays a significant role in various industrial processes and everyday products.
Understanding the basics of formaldehyde involves recognizing its structure and properties. As a colorless gas with a pungent odor, formaldehyde is highly reactive due to its carbonyl group. Its versatility makes it valuable in manufacturing resins, plastics, and even embalming fluids.
In nature, formaldehyde can be found as a byproduct of combustion or metabolic processes. Despite its widespread use, exposure to high levels of formaldehyde can pose health risks. Thus, studying the fundamentals of H2CO is crucial for both scientific research and safety considerations.
Step-by-step guide to drawing the Lewis Structure for H2CO
Interested in mastering the art of drawing Lewis Structures for molecules like H2CO? Let’s dive into a step-by-step guide to help you understand and visualize the arrangement of atoms and electrons.
Start by counting the total number of valence electrons in each element present in H2CO: hydrogen, carbon, and oxygen. Remember, hydrogen only needs two electrons to achieve stability while carbon and oxygen strive for octets.
Next, place the least electronegative atom (usually carbon) at the center. Attach hydrogen atoms first as they can only form single bonds. Oxygen will typically form double bonds due to its higher electronegativity.
Distribute remaining electrons around atoms to fulfill their octet or duet rule. Ensure each atom obeys the octet rule unless it is hydrogen which follows the duet rule.
Double-check your structure for correct bond connections and electron distribution within a stable framework that satisfies all elements’ valence electron requirements.
Common mistakes to avoid when drawing Lewis Structures
When it comes to drawing Lewis Structures, there are some common pitfalls that many students fall into. One mistake to avoid is forgetting to count the total number of valence electrons available for bonding in the molecule. This can lead to an incorrect structure being drawn.
Another mistake is incorrectly placing lone pairs on atoms. Lone pairs should be placed strategically to minimize formal charges and achieve a stable structure. Additionally, make sure not to forget about double or triple bonds when necessary – they play a crucial role in determining the overall shape of the molecule.
Overlooking octet rule violations is also a common error. Remember that certain elements can expand their octets beyond eight electrons in their valence shell.
Don’t forget about resonance structures! Sometimes molecules can have multiple valid Lewis Structures due to resonance, so explore all possible arrangements before finalizing your diagram.
Other uses of Lewis Structures in chemistry
Lewis Structures are not just limited to drawing the arrangement of atoms and electrons in molecules. They have a wide array of applications across various fields within chemistry. One significant use is in predicting molecular geometry, which helps understand how molecules interact with each other. By analyzing Lewis Structures, chemists can also determine the polarity of molecules, crucial for studying chemical reactions.
Furthermore, Lewis Structures play a vital role in explaining the reactivity and stability of compounds. Understanding the electronic configuration through these structures aids researchers in designing new materials with specific properties or enhancing existing ones. Additionally, they are instrumental in organic chemistry for predicting reaction mechanisms and understanding bond formations between different elements.
In biochemistry, Lewis Structures assist scientists in comprehending biological processes at a molecular level, shedding light on pharmaceutical interactions and drug design. The versatility of Lewis Structures extends far beyond basic structural representation, providing valuable insights into the intricate world of chemistry.
Conclusion
Lewis Structures are a fundamental tool in chemistry, providing insights into the bonding and structure of molecules. Understanding how to draw Lewis Structures can greatly enhance your comprehension of chemical compounds like formaldehyde (H2CO). By following a step-by-step guide and being mindful of common mistakes, you can accurately represent the arrangement of atoms and electrons in a molecule.
Remember that Lewis Structures have various applications beyond just drawing them for individual molecules. They can help predict molecular geometry, reactivity, and even aid in understanding complex organic reactions. Embracing this foundational concept will undoubtedly benefit your journey through the world of chemistry.
So next time you encounter H2CO or any other lewis structure for h2co compound, be sure to look out for its Lewis Structure—it might just unlock a whole new level of understanding in your studies or research endeavors. Happy drawing!


![[silent war] taming a tsundere](https://newsipedia.com/wp-content/uploads/2024/04/download-20-1.jpeg)

